10X Genomics suggested protocol for nuclei isolation for the 10X snRNA-Seq workflow
Below is a suggested protocol for nuclei isolation. 10X Genomics has a few demonstrated protocols for sample preparation, and we highly recommend to have a look at them as well.
Buffers
Wash buffer (WB)
| Components | Final concentration | Volume for 1ml |
|---|---|---|
| Tris-HCl (1M, pH 7.4) | 10 mM | 10 µl |
| NaCl (5 M) | 10 mM | 2 µl |
| MgCl2 (1 M) | 3 mM | 3 µl |
| BSA (10 %) | 1% | 100 µl |
| Tween 20 (10 %) | 0.1% | 10 µl |
| Nuclease-free water | - | 875 µl |
Lysis Buffer (LB)
| Components | Final concentration | Volume for 1ml |
|---|---|---|
| Wash buffer | 980 µl | |
| Nonidet P40 Substitute (10%) | 0.1% | 10 µl |
| Digitonin (1%) | 0.01% | 10 µl |
Diluted Nuclei Buffer (DNB)
| Components | Final concentration | Volume for 1ml |
|---|---|---|
| 10X Genomics Nuclei Buffer (20x stock) | 1x | 50 |
| Nuclease-free water | 950 |
Nuclei isolation
Isolate single cell suspension with an appropriate protocol, in a standard 1.5 ml tube. Start with at least 100.000 cells as input, max 1 mio cells.
If cells are FACS-sorted, pre-load 5 µl of cell medium or PBS into the tube before sorting starts
Centrifuge cells at 300 rcf for 5 min at 4°C. [choose the speed appropriate for your cell type]
Remove supernatant. ~5 µl may remain.
Add 100 µl chilled Lysis Buffer (Table 2) and gently pipette mix three times.
Without further incubation, centrifuge directly at 500 rcf for 5-10 min4 at 4°C.
Remove supernatant without disrupting the nuclei pellet.
Add 100 µl chilled Wash Buffer (Table 1) to the tube and gently pipette mix three times. Optionally, simply flick the tube a few times; the nuclei should resuspend easily after 3-4 taps.
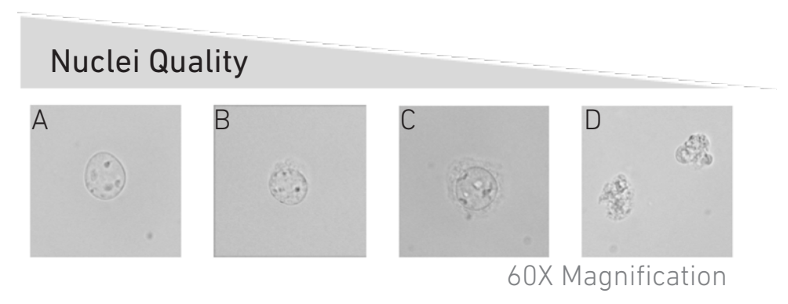
Quality control step: Check nuclei quality under microscope. Mix 8 µl trypan blue (0.4% solution) with 2 µl of the nuclei suspension,5 and transfer the mix to a Cell Counting Chamber for counting nuclei and checking nuclei quality (under a 40x objective, at least). Nuclei should be round with intact nuclear membrane, no aggregates and no debris. For comparison see Figure 1 below. If debris are still present in the sample, a second wash can be performed at this step.
Centrifuge the remaining nuclei suspension at 500 rcf for 5 min at 4°C.
Remove most of the supernatant with a P100/200 pipette, then remove the remaining liquid with a 10µl pipette without touching the bottom of the tube, to avoid dislodging the nuclei pellet.
Resuspend the nuclei pellet in appropriate volume of chilled Diluted Nuclei Buffer (Table 3) , targeting the desired nuclei concentration (ideal 4.000 - 8.000 nuclei/µl)
(Picture taken from 10X Userguide)
Notes
- Expected nuclei recovery is around 25%, so please use an appropriate number of cells as input to achieve the desired number of nuclei.
- It is advised to test different detergent concentrations. For this, lysis buffer can be diluted with wash buffer to 1:5, 1:10, 1:20 or 1:50 for example. (1:10 dilution is a good starting point)
- A short incubation period of 3-5min can be included at this step in case cell lysis is sub-optimal.
- Do not reduce spinning time to less than 5 minutes.
- You can mix 5 µl trypan blue + 5 µl nuclei mix if you have sufficient starting material or only want to check nuclei quality. The more nuclei you can observe, the better you can estimate the quality.
- Alternatively, you can add a DNA-intercalating dye such as propidium iodide to check for nuclei integrity and free floating DNA.